Things must be done right, the first time, especially, when it is related to regulatory conformance & most importantly in stringent environment of pharmaceutical industry where each product runs through myriad tests, checks & controls right from first stage of drug development until it reaches out the consumer.

Enterprises deploy a special group of quality professionals (QP), to review samples & test results, & be utmost sure of product’s safety & quality controls before recording the batch fit for distribution & sales to customers in the market. EU Annex 16 of guidelines for GMP, emphasizes the importance of batch releases through a qualified professional. All imported products manufactured outside of the EU must be batch tested & released before triggering sales within EU member states or members of European Economic Area (EEA).
A batch would not leave the warehouse, until signed off by the QP for meeting all release criteria.
Since Pharma Industry comes under essential commodities due to which in time of such pandemic they have to work in double shifts to produce medicines to fight unprecedented critical times.
Timely release of products for an enterprise, hence, becomes most assertive Quality function task to ensure drugs reach the patients in such challenging times.
Few challenges to address:
- Office presence to review/release a batch
- Delays on timely reach or product launches due to lack of accessibility of information outside office premise
- Existing paper-based activities leading to errors and non-conformance
- Cross functional synchronization-teams working in silos
- Managing To-Do list amongst Quality function
It is imperative for organizations to flexibly bend their strategies & move out from traditional paper-based approach and rely on technology solutions to capitalize on timely launches & reach of medicines to patients without delays & ensuring full compliance
As a response to Covid-19, PharmaNET Quality solution provisions to solve above noted challenges & achieve benefits:
- Transparent visibility across goods receipt, samples, test, review & release status
- Seamless communication across cross functions with timely notifications
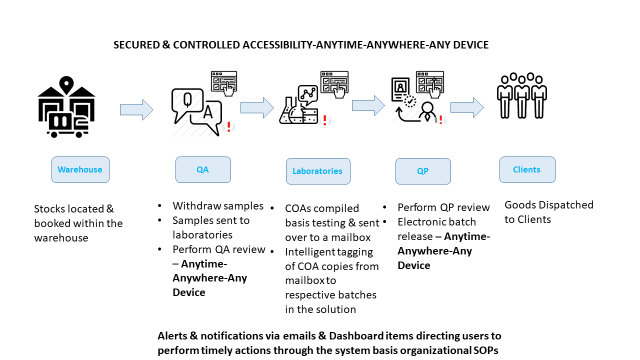
- QA & QP can review and release batches anytime, anywhere, while being at remote places from any device
- Manage COAs, BRC for each batch on a single platform with controlled & secured accessibility
- Timely batch release & stock reach to the market
- Increase in sales revenue & organization growth
With work force scattered across geography & being mobile, ensure quality, safety, efficacy of products with timely reach to the markets leveraging PharmaNET technology solution addressing batch review and release activities meeting all global regulatory compliance.
Refer below, for a broader understanding